Irreversible Photoactivatable Fluorophores 2016–2026
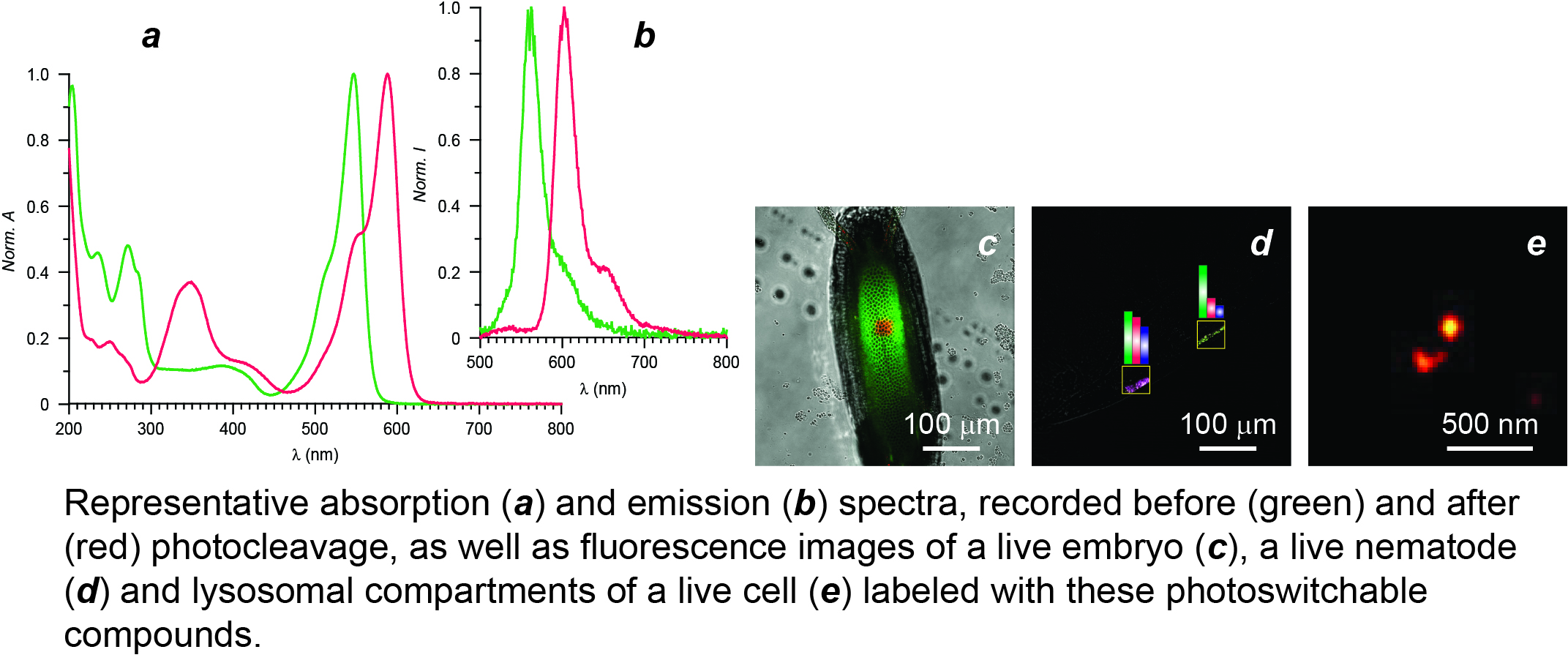
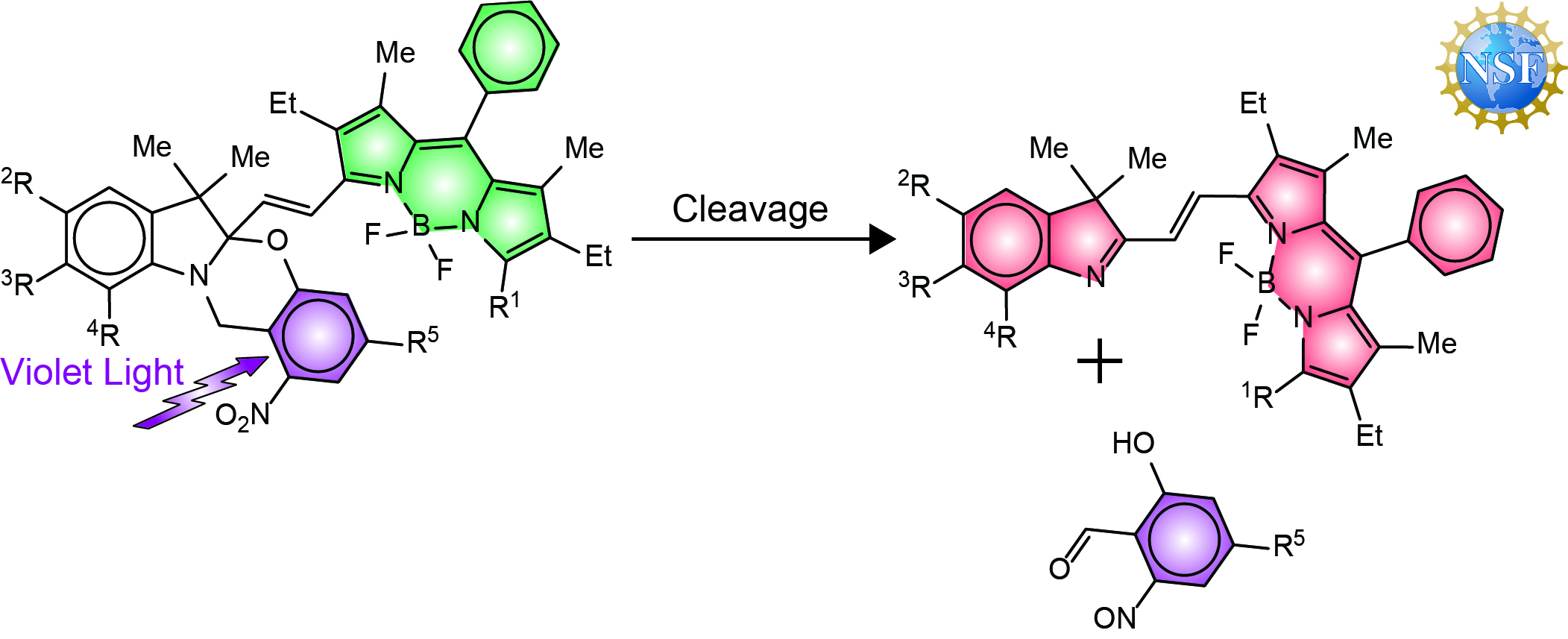 The need for viable analytical methods to monitor dynamic events in live specimens motivated Professor Raymo to re-engineer the structural design of his photoswitchable fluorophores. Specifically, he aimed his research program at the realization of photoresponsive molecules capable of changing the color of their emission in response to biocompatible illumination conditions with the ultimate goal of developing a general strategy to probe dynamics in vivo on the basis of fluorescence measurements. Indeed, current methodologies to follow dynamic processes rely on the bleaching of conventional fluorophores in a defined region of space at a precise interval of time to monitor the subsequent and gradual fluorescence recovery in the bleached area. However, the bleaching step demands harsh irradiation conditions that can cause significant photodamage to biological samples. He envisaged the possibility of overcoming these complications with the photochemical generation, rather than bleaching, of fluorescent probes in live samples, under mild visible illumination, and the subsequent tracking of their translocation. In order to implement these potentially-transformative operating principles, his research group developed a new generation of photoswitchable probes, based on the photoinduced cleavage of an ortho-nitrobenzyl group from a borondipyrromethene (BODIPY) fluorophore, over the course of three funding cycles from the National Science Foundation. These molecules switch irreversibly between states with spectrally-resolved fluorescence (a and b), under mild irradiation with violet light. This unique behavior permits the photoinduced conversion of reactants into products with spatiotemporal control and the subsequent monitoring of their dynamics in real time with the parallel acquisition of time-lapse images in spectrally-resolved detection channels. His research group demonstrated that these photoresponsive compounds allow the tracking of molecular diffusion across the cellular blastoderm of live embryos (c) as well as of the translocation of polymer beads within the intestinal tract of live nematodes (d). Furthermore, the excellent photophysics of their BODIPY component translate into a dramatic improvement in brightness, relative to the original photoswitchable fluorophores, that facilitates single-molecule detection to enhance localization precision. In fact, the dramatic improvement in performance of this new generation of photoswitchable fluorophores enabled the imaging of the lysosomal compartments of live cells with sub-diffraction resolution (e). His research group is currently exploring further structural modifications with the objective of inducing photocleavage with green light and, possibly, avoiding altogether any toxic effects that violet irradiation may have on live samples. Should these efforts be successful, these molecules may evolve into the photoactivatable fluorophores of choice in the biomedical laboratory to allow the investigation of dynamic processes in live organisms and the visualization of nanostructures in live cells.
The need for viable analytical methods to monitor dynamic events in live specimens motivated Professor Raymo to re-engineer the structural design of his photoswitchable fluorophores. Specifically, he aimed his research program at the realization of photoresponsive molecules capable of changing the color of their emission in response to biocompatible illumination conditions with the ultimate goal of developing a general strategy to probe dynamics in vivo on the basis of fluorescence measurements. Indeed, current methodologies to follow dynamic processes rely on the bleaching of conventional fluorophores in a defined region of space at a precise interval of time to monitor the subsequent and gradual fluorescence recovery in the bleached area. However, the bleaching step demands harsh irradiation conditions that can cause significant photodamage to biological samples. He envisaged the possibility of overcoming these complications with the photochemical generation, rather than bleaching, of fluorescent probes in live samples, under mild visible illumination, and the subsequent tracking of their translocation. In order to implement these potentially-transformative operating principles, his research group developed a new generation of photoswitchable probes, based on the photoinduced cleavage of an ortho-nitrobenzyl group from a borondipyrromethene (BODIPY) fluorophore, over the course of three funding cycles from the National Science Foundation. These molecules switch irreversibly between states with spectrally-resolved fluorescence (a and b), under mild irradiation with violet light. This unique behavior permits the photoinduced conversion of reactants into products with spatiotemporal control and the subsequent monitoring of their dynamics in real time with the parallel acquisition of time-lapse images in spectrally-resolved detection channels. His research group demonstrated that these photoresponsive compounds allow the tracking of molecular diffusion across the cellular blastoderm of live embryos (c) as well as of the translocation of polymer beads within the intestinal tract of live nematodes (d). Furthermore, the excellent photophysics of their BODIPY component translate into a dramatic improvement in brightness, relative to the original photoswitchable fluorophores, that facilitates single-molecule detection to enhance localization precision. In fact, the dramatic improvement in performance of this new generation of photoswitchable fluorophores enabled the imaging of the lysosomal compartments of live cells with sub-diffraction resolution (e). His research group is currently exploring further structural modifications with the objective of inducing photocleavage with green light and, possibly, avoiding altogether any toxic effects that violet irradiation may have on live samples. Should these efforts be successful, these molecules may evolve into the photoactivatable fluorophores of choice in the biomedical laboratory to allow the investigation of dynamic processes in live organisms and the visualization of nanostructures in live cells.
Support
- “Photochemical Strategies to Monitor Cellular Dynamics in Living Organisms with Supramolecular Assistance”: National Science Foundation, CHE-1505885 (PI: F. M. Raymo), $ 450,000, 01/15/16–12/31/18
- “Photochemical Strategies to Activate Far-Red Fluorescence with Green Light”: National Science Foundation, CHE-1954430 (PI: F. M. Raymo; Co-PIs: H. F. Zhang, Y. Zhang), $ 580,000, 08/15/20–07/14/23
- “Collaborative Research: Spectral Discrimination of Single Molecules with Photoactivatable Fluorescence”: National Science Foundation, CHE-2246547 (PI: F. M. Raymo; Co-PI: Y. Zhang), $ 409,929, 09/01/23–08/31/26
Selected Articles
- “Photoactivatable BODIPYs Designed to Monitor the Dynamics of Supramolecular Nanocarriers”: Y. Zhang, S. Swaminathan, S. Tang, J. Garcia-Amorós, M. Boulina, B. Captain, J. D. Baker, F. M. Raymo, J. Am. Chem. Soc., 2015, 137, 4709–4719
- “A Photoswitchable Fluorophore for the Real-Time Monitoring of Dynamic Events in Living Organisms”: Y. Zhang, S. Tang, L. Sansalone, J. D. Baker, F. M. Raymo, Chem. Eur. J., 2016, 22, 15027–15034
- “A Photoactivatable Light Tracer”: X. Liu, Y. Zhang, J. D. Baker, F. M. Raymo, J. Mater. Chem. C, 2017, 5, 12714–12719
- “Photochemical Barcodes”: S. Tang, Y. Zhang, P. Dhakal, L. Ravelo, C. L. Anderson, K. M. Collins, F. M. Raymo, J. Am. Chem. Soc., 2018, 140, 4485−4488
- “A Photoactivatable Far-Red/Near-Infrared BODIPY to Monitor Cellular Dynamics in Vivo“: L. Sansalone, S. Tang, J. Garcia-Amorós, Y. Zhang, S. Nonell, J. D. Baker, B. Captain, F. M. Raymo, ACS Sens., 2018, 3, 1347–1353
- “Far-Red Photoactivatable BODIPYs for the Super-Resolution Imaging of Live Cells”: Y. Zhang, K.-H. Song, S. Tang, L. Ravelo, J. Cusido, C. Sun, H. F. Zhang, F. M. Raymo, J. Am. Chem. Soc., 2018, 140, 12741−12745
Selected Reviews
- “Fluorescence Activation with Switchable Oxazines”: Y. Zhang, S. Tang, E. R. Thapaliya, L. Sansalone, F. M. Raymo, Chem. Commun., 2018, 54, 8799–8809
- “Live-Cell Imaging at the Nanoscale with Bioconjugatable and Photoactivatable Fluorophores”: Y. Zhang, F. M. Raymo, Bioconjugate Chem., 2020, 31, 1052–1062
- “BODIPYs with Photoactivatable Fluorescence”: Y. Zhang, Y. Zheng, Y. Meana, F. M. Raymo, Chem. Eur. J., 2021, 27, 11257–11267
- “Photoactivatable Fluorophores for Bioimaging Applications”: Y. Zhang, Y. Zheng, A. Tomassini, A. K. Singh, F. M. Raymo, ACS Appl. Opt. Mater., 2023, 1, 640–651